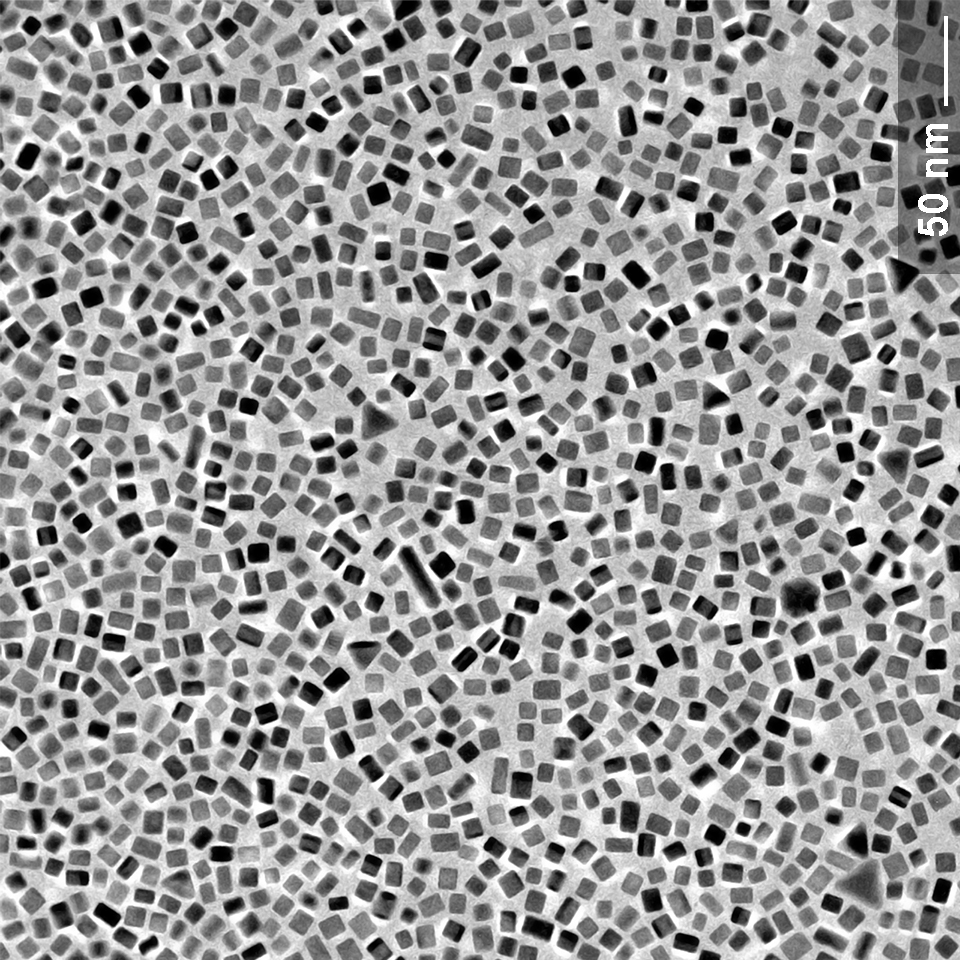
Nanoscientifica Palladium Cubes - Free Sample*
This product is no longer available.
Volumes: 10 mL
Size: 10 nm
- Appearance: Black colloidal suspension
- Solvent carrier: Water (Standard). It can be dispersed in Ethanol, Iso-propanol, Glycols, Polar protic solvents.
- Concentration: ≈ 0.01 wt%
- Transport Temperature: 5-30 ºC
- Stock Temperature: 4 ºC. Do not freeze
*We are a small startup, therefore, a small shipping and handling fee will be added.
Description
Sharp cubes of Pd nanocrystals with an average edge size below 10 nm and narrow size distribution are also now available on a large scale though NanoScientifica Scandinavia. Our unique technology provides an ideal toolkit for the realization of novel and more efficient catalysts, which is valuable to the industry. Our overall goal is to bring shaped Platinum Group Metals, PGM, (platinum, palladium, rhodium, iridium, ruthenium, and osmium) Nanoparticles to the catalyst market, automotive emission control (which accounts to 65 % of the gross world demand of platinum and palladium), catalysis of chemicals (e.g., pharmaceutical compounds) conducted in liquid and gas phase, and future fuel cell technologies.
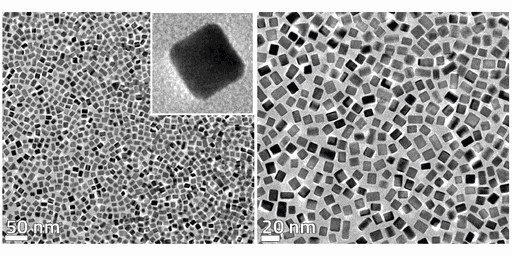
Structural features
By decreasing the size of metal particles down to nanoscale, more atoms are exposed to the crystalline facets (larger surface area) encompassed by local rearrangement of the atoms in restrained geometrical space. This entanglement between size and shape of metal nanocrystal governs, for instance, their catalytic performance. PGM nanoparticles enclosed by specific facets abruptly enhance the catalytic activity of chemical processes in liquid or vapor phases and improve the product selectivity in comparison to particles having the same or smaller sizes but with indistinct shape. For instance, Pd nanocubes enclosed by {100} facets have shown greater catalytic activity for formic oxidation, oxygen reduction, pyrrole hydrogenation, and Suzuki coupling. NanoScientifica Pd Cubes contains >98% {100} facets and can be easily combined with high surface area metallic oxide or carbon-based microparticles and exploited as a catalyst for the synthesis of pharmaceutical compounds, fuel cells, hydrogen sensing, and storage, etc. Our standard solution is to ship the particles in aqueous solution, please inquire about other solvents or solid support.
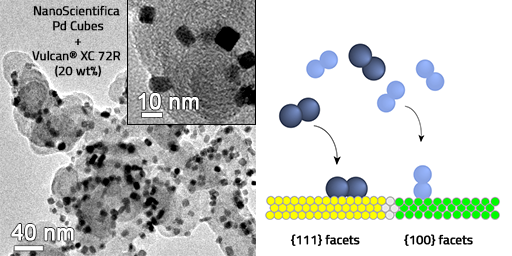
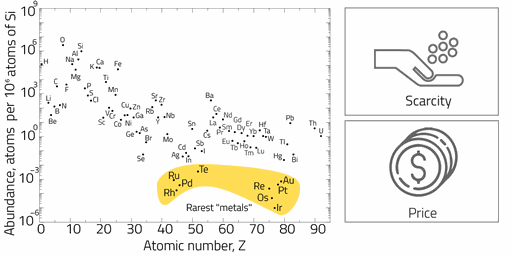
Scarce metals
Resource scarcity is one of the most critical global challenges. Based on their crustal concentrations in mineable deposits, PGM are scarce and much less productive than those comprising more common metals. PGMs are valued for their myriad industrial applications including the manufacture of automotive catalytic converters and hydrogen production (e.g. fuel cells). Current state-of-the-art Platinum Group catalysts are mostly built on poor shape and size control small nanoparticles, which usually provides poor reaction selectivity and limited performance. More strict emission control policies and increasingly demand for cleaner energy platforms are imposing pressure on the use of catalysts with higher loadings of PGM materials. As thumb role, the greater the geological scarcity of the mineral, the earlier the price increase will start while exhaustively consumed. NanoScientifica Scandinavia is proud to empower the PGM catalyst market by large-scale distribution of competitive morphology controlled nanocatalyst.
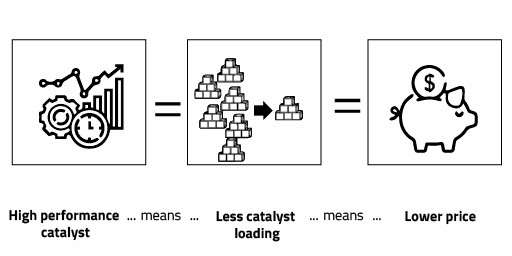
Core advantages
In the scientific literature, it is well established that small and geometrically controlled nanocatalyst shows a higher catalytic performance when compared with particles attaining indistinct shape. NanoScientifica Scandinavia is fully engaged into maximize resource utilization of scarce PGM materials by enabling the production of highly catalytic materials. Our utmost target is the fabrication of industrial nanocatalyst coming 100% from recycled metal sources in the next 2 years.
Physical-Chemical Properties
Ranking in order of abundance in earth crust | 70 |
Mean content in earth crust | 0.015 ppm (g/tonne) |
Mean content in oceans | - |
Residence time in oceans | - |
Radii of atoms (ppm) | Atomic: 140Covalent: 131 |
Density (g cm-3) | 12.02 |
Molar volume (cm3) | 8.85 |
Specif ic heat cp at 298 K (J K–1 kg–1) | 244 |
Thermal conductivity (Wm–1K–1) | 173 K: 72273 K: 72373 K: 79973 K: 93 |
Coefficient of linear expansion (K–1) | 100 K: 8 · 10-6293 K: 11.8· 10-6500 K: 13.2 · 10-6800 K: 14.5 · 10-6 |
Resistivity (nΩm) | 78 K: 17.3273 K: 100373 K: 138573 K: 210973 K: 3301473 K: 420 |
Mass magnetic susceptibility (m3 kg-1) | +67.0 · 10-9 |
Youngs modulus (G Pa) | 122 |
Shear modulus (G Pa) | 44 |
Bulk modulus (G Pa) | 180 |
Poissons ratio | 0.39 |
Enthalpy of fusion ΔHfus at melting point (kJmol-1) | 16.7 |
Enthalpy of vaporization ΔHvap at boiling point (kJmol-1) | 390 |
Enthalpy of atomization ΔHat at 298 K (kJmol-1) | 378 |
Entropy S0 at 298 K (J K-1mol-1) | 37.57 |
Molar heat capacity Cp at temperature K (J K-1mol-1) | 100 K: 17.9 |
Health and Safety Information
Signal Word | Warning | |
|---|---|---|
Hazard Statements | H400-H410 | |
Hazard Codes | Xn,N | |
Precautionary Statements | P273-P391-P501a | |
Flash Point | Not applicable | |
Risk Codes | 22-36/38-50/53 | |
Safety Statements | 22-60-61 | |
RTECS Number | GL8900000 | |
Transport Information | UN 3077 9 / PGIII | |
WGK Germany | 3 | |
GHS Pictograms |
This is a preview of the recently viewed products by the user.
Once the user has seen at least one product this snippet will be visible.
Once the user has seen at least one product this snippet will be visible.